Cyclops®
Pre-filled
dry Powder inhaler

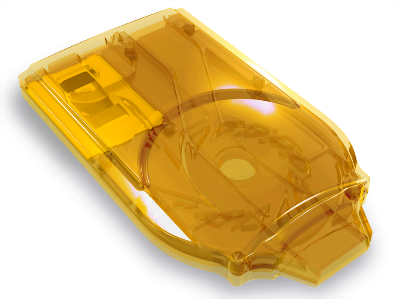
Cyclops® is a state-of-the-art, easy to use, pre-filled DPI that is developed by PureIMS for inhalation powders.
Because of its simple yet
sophisticated proprietary design it can be produced in a cost-effective way.
Cyclops® is available in single & multi use versions.
Upon inhalation it uses the patient’s breath to disperse the dry powder formulation into small particles appropriately sized for deep lung deposition. Cyclops® has several advantages compared to standard-of-care DPIs across key therapeutic areas. These attributes enable the hygienic and effective use on a worldwide scale. One product, Colistin Cyclops®, is already available to patients and reimbursed under a named patient regimen for the treatment of cystic fibrosis.
HIGHLY PREFERRED BY PATIENTS
LATEST NEWS

QA officer
Locatie: Roden, Nederland Omvang: 0.4-0.6fte In verband met de uitbreiding
February 22, 2024

PureIMS gears up for abbreviated registration of Levodopa Cyclops® against OFF episodes in Parkinson’s disease with the successful completion of a comparative pharmacokinetic study
Roden, the Netherlands, January 18, 2024 PureIMS, a pharmaceutical company
January 18, 2024

PureIMS winning the LIFE Science Innovation Award!
Tuesday September 26 was an exciting day for the PureIMS team. Not
September 27, 2023

PureIMS initiates dose-finding study with Levodopa Cyclops™ dry powder inhaler
Following a successful funding round in April of this year
August 23, 2023
PureIMS secures new investment round to develop lead inhalation product and facilitate partnering activities
PureIMS today announced it has secured a new investment round.
April 3, 2023